Learn Anywhere, Anytime
Award Winning AI-Based Learning Solutions
Join hundreds of educators and trainers on Paradiso Solutions.
Ready to experience Paradiso LMS?
Learn Anywhere, Anytime
Join hundreds of educators and trainers on Paradiso Solutions.
Ready to experience Paradiso LMS?
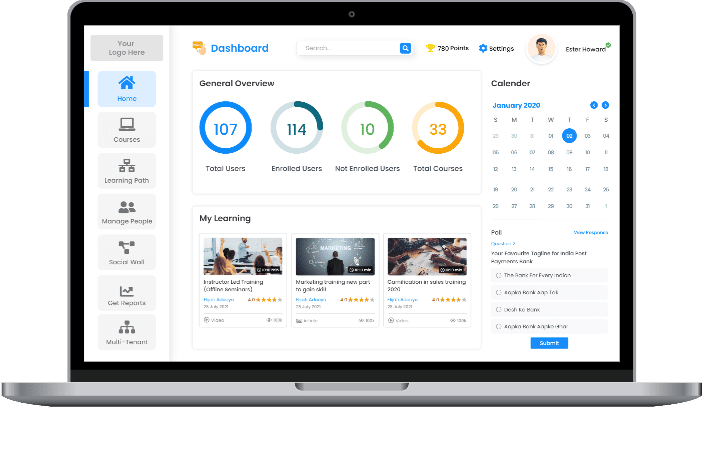
At Paradiso Solutions, we understand that each industry has unique learning requirements. Our eLearning software is designed to be fully customizable, offering an array of features and seamless integrations to address your specific needs. Say goodbye to juggling multiple platforms and elevate your productivity with our all-in-one, adaptable solution.
Witness enhanced learning experience with personalized learning solutions in AI powered LMS
Create engaging and Interactive content with ease using our eLearning authoring tool

Bespoke eLearning courses that fit your unique needs, ensuring engaging and effective content for training
CogniSpark AI, your personalized AI assistant that can be acts as a tutor to solve any queries instantly
Paradiso Solutions offers a robust suite of eLearning tools, including LMS, LXP, Authoring Tools, Microlearning, AI Tutors, and KMS. Our technology ensures that all your learning needs are met with cutting-edge solutions designed to enhance the overall learning experience.
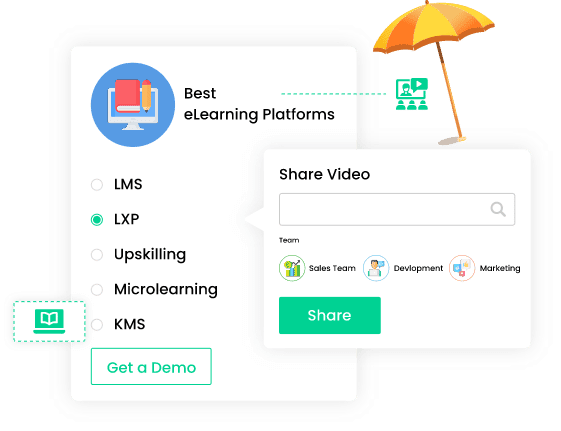

Discover a wealth of features designed to cultivate an exceptional eLearning environment. Our platforms offer extensive customization options, allowing you to tailor features to fit your specific needs and budget.
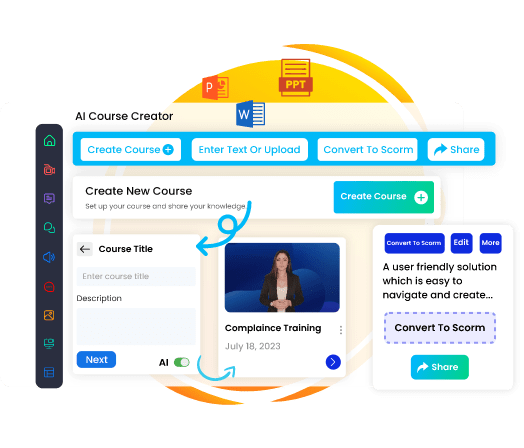
Our AI-driven authoring tool streamlines content creation with an intuitive interface and advanced features. The integrated AI Media Studio enables quick generation of articles, social media posts, pictures, videos, and voice-overs in 50+ languages. Create impactful, diverse content effortlessly with just one application.
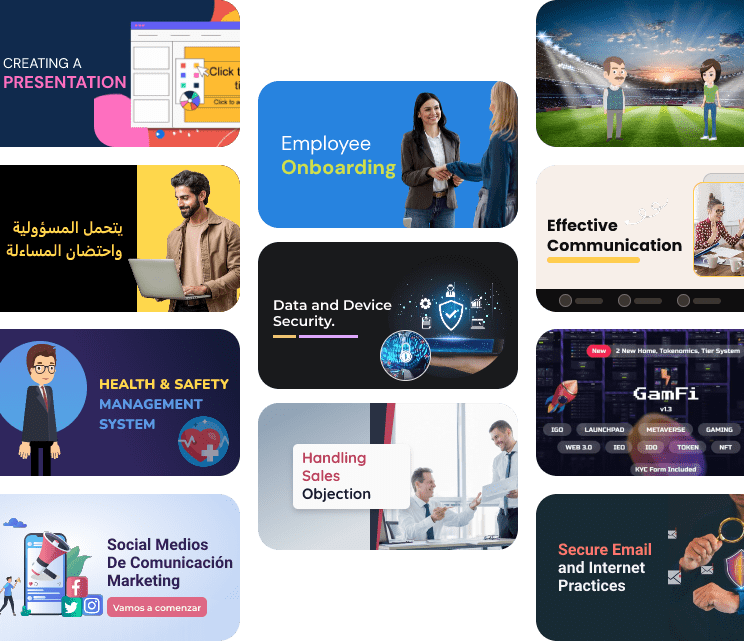
We provide tailor-made eLearning solutions to fit your specific organizational requirements. From bespoke course development to LMS consulting along with managed training services for leading enterprises, ensuring solutions that fit your specific requirements.
Our platform ensures the highest level of data protection with ISO certification and compliance with SOC 2 and HIPAA standards. Trust us to safeguard your information with the highest security measures.
Discover achievements that make us proud!
#GET FREE LMS DEMO
Discover how Paradiso LMS can revolutionize learning, training, and growth within your organization.
Book Your Personalized LMS Demo Today!
Get answers to your specific questions and find out Why Paradiso Solutions is the right choice for your organization.